Earth's atmosphere is like a protective blanket that surrounds our planet and is composed of a mix of gases. While many trace gases are present in tiny amounts, most of the air we breathe comes from just a few major ones. Each plays a vital role in keeping life on Earth thriving. Oxygen, for example, is crucial for almost all living organisms because it powers respiration. Carbon dioxide, though present in much smaller amounts, helps regulate Earth's temperature and is essential for photosynthesis in plants. But do you know which gas is the most abundant of all? It makes up about 80% of the entire atmosphere and is a fundamental building block of proteins in every living organism. In this article, we'll explore the makeup of our atmosphere and reveal the gas that truly dominates it.
Which Gas Is Most Abundant in Earth's Atmosphere?

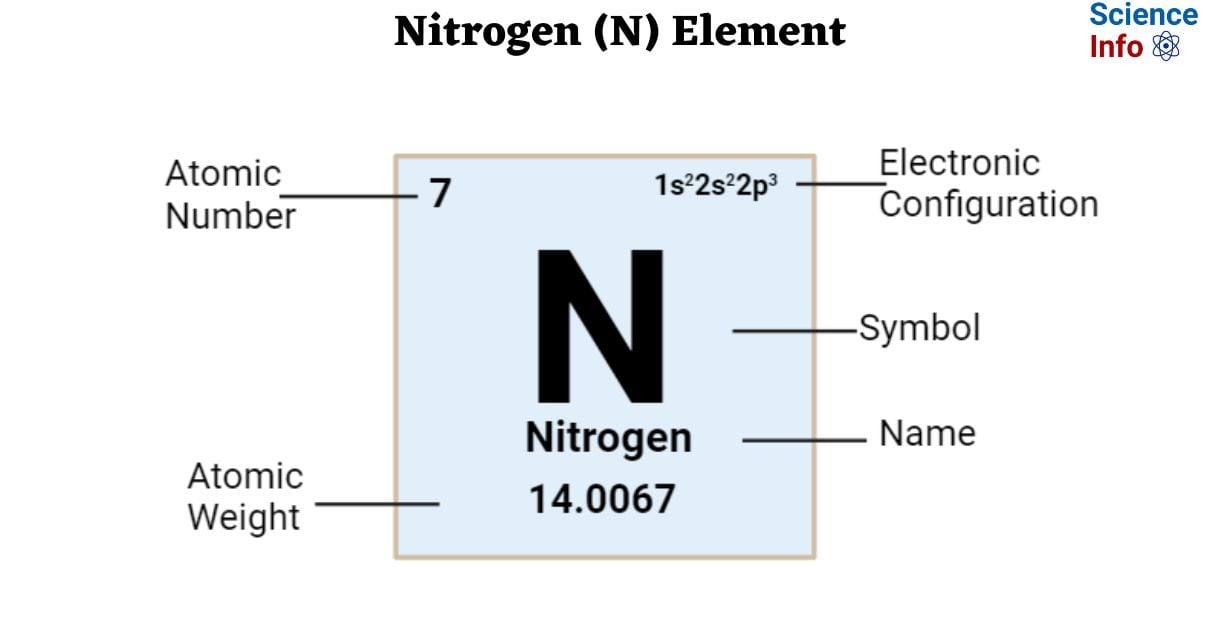
The most abundant gas in Earth's atmosphere is nitrogen (N₂), which makes up approximately 78% of the total volume of dry air. This overwhelming abundance, compared to about 21% for oxygen, is primarily due to nitrogen's chemical structure. The nitrogen molecule has a strong triple bond between its two atoms, which makes it highly unreactive, or "inert," under normal conditions.
Because it rarely reacts with other elements or compounds on the Earth's surface, very little of it is removed from the atmosphere over time. It has been released through processes such as volcanic eruptions and the decay of organic matter for billions of years, allowing it to accumulate easily. Nitrogen's stability and resistance to chemical change are the key reasons for its dominant presence.
10 Lesser-known Facts about Nitrogen
- In its gaseous form, nitrogen has no colour, smell, or taste.
- Every living thing needs nitrogen to build proteins and DNA.
- Plants cannot use atmospheric N₂ directly; special bacteria "fix" it into usable compounds in the soil.
- It acts as a diluent, slowing down chemical reactions like rusting and fire (combustion) caused by oxygen.
- In its liquid state, it is extremely cold (boiling point: -196°C or -320°F) and is used in cryogenics.
- It's often pumped into food bags (like chips) to replace the oxygen, preventing spoilage and keeping the food fresh.
- Nitrogen gas is rapidly generated in car airbags to inflate them almost instantly during a collision.
- Nitrogen compounds, such as those in TNT, are highly reactive and are used to make explosives.
- Nitrogen ions are responsible for the blue-violet and deep violet colours seen in the aurora borealis (Northern Lights).
- While dominant in Earth's atmosphere, hydrogen is the most abundant element in the entire universe.
Which Gas Is Least Abundant in the Earth's Atmosphere?

The gas that is generally considered the least abundant stable noble gas in Earth's atmosphere is xenon (Xe). It is present only in trace amounts, at about 0.086 parts per million by volume. This extreme scarcity, often referred to as the "missing xenon problem," is due to a few factors. First, xenon is a heavy element and was not produced in abundance in the early universe, so Earth received very little primordial xenon.
Second, unlike lighter gases like argon (which is continuously produced by the radioactive decay of potassium-40), xenon has no significant natural source replenishing it in the atmosphere. Current theories suggest that a large portion of the Earth's original xenon may have chemically bonded and become trapped within minerals deep inside the planet's crust, preventing it from outgassing into the atmosphere.
10 Lesser-known Facts about Xenon
- Xenon's name comes from the Greek word xenos, meaning "stranger" or "foreign."
- Xenon is about 4.5 times denser than the air you breathe.
- Despite being a noble gas, xenon was the first noble gas found to form true chemical compounds (like xenon fluorides).
- Xenon is used as a propellant for high-efficiency ion thrusters on some spacecraft (like NASA's Dawn probe).
- It produces a brilliant, bright blue-white flash in high-speed camera flash tubes and car headlights.
- Xenon gas is a safe and effective general anaesthetic with a rapid onset and recovery.
- It works by inhibiting specific brain receptors (NMDA receptors).
- Xenon is a significant byproduct of nuclear fission in reactors, with isotopes like Xenon-135 acting as a "neutron poison."
- Liquid xenon is used in dark matter detectors because it emits light when charged particles pass through it.
- Scientists have created solid xenon with a faint blue hue by applying extreme pressure.
Comments
All Comments (0)
Join the conversation