Key Points
- Diamond is pure carbon (C) formed under extreme pressure in Earth's mantle.
- Diamonds are found in kimberlite pipes, glacial tills, and alluvial gravels.
- Since 1955, diamonds have been made synthetically for industrial uses.
Diamond is the only gem with a single element. Diamonds are formed in the Earth’s mantle, at depths exceeding 120 km (75 miles), under extreme temperatures and pressures.
Over a billion years, the composition of a diamond forms a crystal structure that makes a diamond the hardest known natural substance. Volcanic eruptions bring them to the surface.
Diamonds are mostly found in three types of diamond-bearing deposits: kimberlite pipes, glacial tills, and alluvial gravels.
The kimberlite pipes (primary source) are volcanic conduits that bring diamonds from deep within the Earth’s mantle to the surface.
Diamonds eroded from kimberlites are washed, carried and deposited in rivers (alluvial) and glacial till.
Diamonds come in different forms and colours. It can be colourless to black. Diamonds can be translucent, transparent, or opaque. Since 1955, diamonds have also been made synthetically.
The hardness, sparkle, and brilliance of diamonds make them the most popular gemstone. Known for their high hardness and thermal conductivity, diamonds are used in major industrial applications such as cutting and polishing tools.
Isn’t that some interesting trivia you learnt about diamonds? In our previous articles, we explored the IUPAC (chemical names) of salt, chalk, vinegar, toothpaste, alum (fitkari), and many more.
Let us now learn the chemical name and formula, along with the properties and uses of diamond in everyday life.
What is the chemical name and formula for diamond?
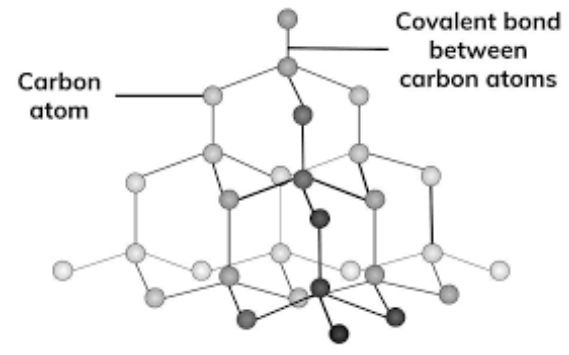
The chemical name for diamond is Carbon, with the chemical formula C.
Diamond is a pure, crystalline form (allotrope) of the element Carbon.
Diamond is formed deep in the Earth’s mantle when carbon crystallises under immense pressure and temperature to form a crystal structure called diamond cubic.
Diamonds have the greatest number of atoms per unit volume, which makes them both the hardest and the least compressible.
Each carbon atom in a diamond is attached to four other carbon atoms by strong covalent bonds, forming a tetrahedral structure (sp3 hybridisation).
Source: Wikipedia
Physical Properties of Diamond
Hardness: Hardest natural substance (Mohs 10)
Strength: High Compression (though can break from impact)
Density: High, around 3.5 g/cm3
Melting point: Extremely high (around 4000 degrees Celsius)
Optical: High refractive index
Solubility: Insoluble in water
Structure: Cubic, each carbon atom bonded to four others
Colour: Pure diamond is colourless
Chemical Properties of Diamond
Composition: Pure carbon
Corrosion: High resistance to chemical corrosion
Thermal: Excellent thermal conductor
Electrical Conductivity: Poor
Source: Wikipedia
Uses of Diamond
Diamonds are not just used in jewellery. Their extreme hardness and thermal conductivity make them vital for industrial, construction, electronics, optics, medical, and scientific purposes. Diamonds are also used in high-end speakers, engraving on granite and quartz, and micro-bearing in watches and devices.
Below have a look at the uses of diamond:
Industrial and Construction
- Cutting and grinding
- Polishing
- Wire drawing
Electronics and Optics
- Heat Sinks (due to thermal conductivity, helps dissipate heat in high-power electronics)
- Windows and lenses (used in lasers, X-ray machines, and spectrometers)
- Quantum sensors and devices
Medical and Scientific
- Surgical instruments
- Research (high-pressure material studies)
You may also like...
Enter your Blink text here...
Comments
All Comments (0)
Join the conversation