CBSE Class 10th Science Exam: The CBSE Class 10 Science Compartment Board Exam 2025 is scheduled for tomorrow, July 17, 2025, and students are in the final phase of their preparation. To assist students with quick and effective last-minute revision, we have curated the Top 50 MCQs with Answers, covering all important topics from the CBSE Class 10 Science syllabus. These multiple-choice questions will help students strengthen their conceptual understanding and boost their confidence before appearing for the exam. Make sure to solve these MCQs and check the solutions to ensure you are well-prepared to score high in the CBSE Class 10 Science Exam 2025.
These MCQ's are the last-minute life saviour for students preparing for the exam a day before. They should read them thoroughly and also check the answers for better revision. With this, students who were not able to clear the previous exam can score well in this to get promoted to the next class
Also Check|
- CBSE Class 10 Science Last Day Tips, Key Topics & Formulas for Quick Revision
- CBSE Class 10 Science Answer Writing Tips To Score Full Marks in 2025 Exam
CBSE Class 10 Science Paper: Key Highlights
| Particulars | Details |
| Exam Conducting Body | Central Board of Secondary Education (CBSE) |
| Subject | Science |
| Mode of Exam | Offline |
| Exam Duration | 3 Hours |
| Medium of Exam | English / Hindi |
| Type of Questions | MCQs, Short and Long Answer Type Questions |
| Theory Marks | 80 |
| Internal Assessment | 20 |
| Total Marks | 100 |
| Passing Marks | 33% in aggregate |
Also, check
COMPLETE CBSE Class 10th Science Study Material 2025
CBSE Class 10th Science Exam Top 50 MCQs with Answers
1. What type of reaction is represented by the following equation? 2Mg + O2 → 2MgO
a) Combination Reaction
b) Decomposition Reaction
c) Displacement Reaction
d) Redox Reaction
Answer: Combination Reaction
2. Which of the following is an example of a double displacement reaction?
a) NaCl + AgNO3 → AgCl + NaNO3
b) 2H2 + O2 → 2H2O
c) Zn + HCl → ZnCl2 + H2
d) CaCO3 → CaO + CO2
Answer: NaCl + AgNO₃ → AgCl + NaNO₃
3. What is the pH of pure water?
a) 5
b) 7
c) 9
d) 11
Answer: 7 (b) (pH of pure water)
4. Which of the following is a strong acid?
a) Acetic acid
b) Citric acid
c) Hydrochloric acid
d) Carbonic acid
Answer: Hydrochloric acid
5. Which metal is the best conductor of electricity?
a) Iron
b) Copper
c) Silver
d) Aluminium
Answer: Silver (c) (Best conductor of electricity)
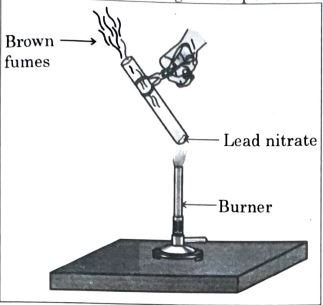
6. The emission of brown fumes in the given experimental setup is due to
(a) the thermal decomposition of lead nitrate which produces brown fumes of nitrogen dioxide.
(b) thermal decomposition of lead nitrate which produces brown fumes of lead oxide.
(c) oxidation of lead nitrate forming lead oxide and nitrogen dioxide.
(d) oxidation of lead nitrate forming lead oxide and oxygen.
Answer: (a) the thermal decomposition of lead nitrate which produces brown fumes of nitrogen dioxide.
7. The general formula of alkane is:
a) CnH2n
b) CnH2n+2
c) CnH2n-2
d) CnH2n+1
Answer: CₙH₂ₙ₊₂ (b) (General formula of alkane)
8. The functional group present in aldehyde is:
a) -OH
b) -COOH
c) -CHO
d) -CO
Answer: -CHO (c) (Functional group of aldehyde)
9. Which part of the plant is responsible for photosynthesis?
a) Root
b) Stem
c) Leaf
d) Flower
Answer: Leaf (c) (Responsible for photosynthesis)
10. In human digestion, which enzyme breaks down starch?
a) Pepsin
b) Trypsin
c) Amylase
d) Lipase
Answer: Amylase (c) (Breaks down starch)
11. Which hormone regulates blood sugar levels?
a) Thyroxine
b) Insulin
c) Adrenaline
d) Estrogen
Answer: Insulin (b) (Regulates blood sugar levels)
12. What is the functional unit of the nervous system?
a) Nephron
b) Neuron
c) Alveoli
d) Axon
Answer: Neuron (b) (Functional unit of the nervous system)
13. What is the mode of reproduction in bacteria?
a) Budding
b) Binary fission
c) Fragmentation
d) Sporulation
Answer: Binary fission (b) (Mode of reproduction in bacteria)
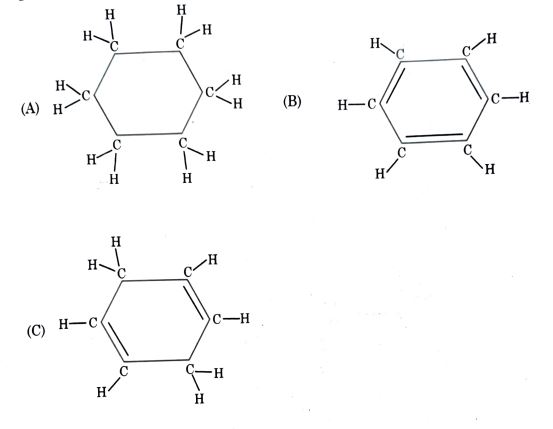
14. Consider the structures of the three cyclic carbon compounds A, B, and C given below and select the correct option from the following:
(Diagrams of compounds A, B, and C as shown in the image)
Options:
(a) A and C are isomers of hexane and B is benzene.
(b) A is an isomer of hexane, B is benzene and C is an isomer of hexene.
(c) A is a saturated cyclic hydrocarbon and B and C are unsaturated cyclic hydrocarbons.
(d) A is cyclohexane and B and C are the isomers of benzene.
Answer: (c) A is a saturated cyclic hydrocarbon and B and C are unsaturated cyclic hydrocarbons.
15. The scientist who proposed the laws of inheritance is:
a) Darwin
b) Lamarck
c) Mendel
d) Pasteur
Answer: Mendel (c) (Proposed laws of inheritance)
16. What is the unit of inheritance?
a) DNA
b) Gene
c) Chromosome
d) Nucleus
Answer: Gene (b) (Unit of inheritance)
17. The focal length of a convex lens is:
a) Positive
b) Negative
c) Zero
d) Infinity
Answer: Positive (a) (Focal length of a convex lens)
18. The mirror used in car side mirrors is:
a) Plane mirror
b) Concave mirror
c) Convex mirror
d) Cylindrical mirror
Answer: Convex mirror (c) (Used in car side mirrors)
19. What is the cause of dispersion of light?
a) Reflection
b) Refraction
c) Scattering
d) Diffraction
Answer: Refraction (b) (Cause of dispersion of light)
20. The part of the human eye that controls the amount of light entering is:
a) Retina
b) Lens
c) Iris
d) Optic nerve
Answer: Iris (c) (Controls the amount of light entering the eye)
21. What type of reaction occurs when iron reacts with oxygen to form rust?
a) Combination Reaction
b) Decomposition Reaction
c) Displacement Reaction
d) Redox Reaction
Answer: a) Combination Reaction
22. Which of the following is an example of a displacement reaction?
a) Zn + CuSO4 → ZnSO4 + Cu
b) 2Mg + O2 → 2MgO
c) CaCO3 → CaO + CO2
d) NaOH + HCl → NaCl + H2O
Answer: a) Zn + CuSO4 → ZnSO4 + Cu
23. The process of breaking down a compound into simpler substances using heat is called:
a) Combination Reaction
b) Displacement Reaction
c) Decomposition Reaction
d) Neutralization Reaction
Answer: c) Decomposition Reaction
24. What is the pH of a neutral solution?
a) 1
b) 7
c) 14
d) 0
Answer: b) 7
25. Which gas is released when an acid reacts with a metal?
a) Oxygen
b) Nitrogen
c) Hydrogen
d) Carbon dioxide
Answer: c) Hydrogen
26. Which of the following acids is present in vinegar?
a) Hydrochloric acid
b) Acetic acid
c) Nitric acid
d) Sulfuric acid
Answer: b) Acetic acid
27. Which non-metal is a good conductor of electricity?
a) Sulfur
b) Phosphorus
c) Graphite
d) Iodine
Answer: c) Graphite
28. The metal that does not react with dilute hydrochloric acid is:
a) Zinc
b) Copper
c) Magnesium
d) Iron
Answer: b) Copper
29. Which process is used for the prevention of rusting?
a) Galvanization
b) Fermentation
c) Sublimation
d) Filtration
Answer: a) Galvanization
30. What is the molecular formula of methane?
a) CH3
b) CH4
c) C2H6
d) C3H8
Answer: b) CH4
31. The functional group present in alcohols is:
a) -OH
b) -COOH
c) -CHO
d) -COO-
Answer: a) -OH
32. The process of converting vegetable oil into solid fat is called:
a) Hydrogenation
b) Saponification
c) Fermentation
d) Hydrolysis
Answer: a) Hydrogenation
33. Which organelle is known as the powerhouse of the cell?
a) Nucleus
b) Mitochondria
c) Ribosome
d) Golgi apparatus
Answer: b) Mitochondria
34. The site of photosynthesis in plant cells is:
a) Mitochondria
b) Chloroplast
c) Ribosome
d) Nucleus
Answer: b) Chloroplast
35. What is the main excretory product in humans?
a) Ammonia
b) Urea
c) Uric acid
d) Carbon dioxide
Answer: b) Urea
36. The hormone responsible for growth in plants is:
a) Cytokinin
b) Auxin
c) Gibberellin
d) Abscisic acid
Answer: b) Auxin
37. Which part of the brain controls voluntary actions?
a) Medulla
b) Cerebellum
c) Cerebrum
d) Hypothalamus
Answer: c) Cerebrum
38. Reflex actions are controlled by:
a) Brain
b) Spinal cord
c) Heart
d) Lungs
Answer: b) Spinal cord
38. The mode of reproduction in amoeba is:
a) Budding
b) Binary fission
c) Fragmentation
d) Vegetative propagation
Answer: b) Binary fission
39. Which hormone controls the menstrual cycle in females?
a) Testosterone
b) Estrogen
c) Insulin
d) Thyroxine
Answer: b) Estrogen
40. Who is known as the father of genetics?
a) Charles Darwin
b) Gregor Mendel
c) Louis Pasteur
d) Watson and Crick
Answer: b) Gregor Mendel
41. Which of the following is an example of a dominant trait in humans?
a) Blue eyes
b) Attached earlobes
c) Curly hair
d) Straight hair
Answer: c) Curly hair
42. The mirror used in headlights of vehicles is:
a) Concave mirror
b) Convex mirror
c) Plane mirror
d) Cylindrical mirror
Answer: a) Concave mirror
43. Which lens is used to correct myopia?
a) Convex lens
b) Concave lens
c) Bifocal lens
d) Cylindrical lens
Answer: b) Concave lens
44. The unit of refractive index is:
a) No unit
b) Meter
c) Hertz
d) Joule
Answer: a) No unit
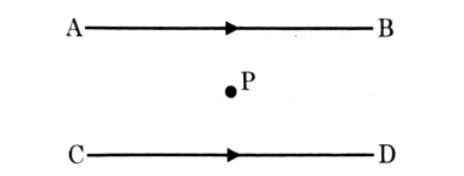
45. The resultant magnetic field at point 'P' situated midway between two parallel wires (placed horizontally) each carrying a steady current I is
(a) in the same direction as the current in the wires.
(b) in the vertically upward direction.
(c) zero
(d) in the vertically downward direction.
46. Consider the following statements:
(i) The sex of a child is determined by what it inherits from the mother.
(ii) The sex of a child is determined by what it inherits from the father.
(iii) The probability of having a male child is more than that of a female child.
(iv) The sex of a child is determined at the time of fertilisation when male and female gametes fuse to form a zygote.
The correct statements are:
(a) (i) and (iii)
(b) (ii) and (iv)
(c) (iii) and (iv)
(d) (i), (iii) and (iv)
Answer: (b) (ii) and (iv)
47. Which one of the following organs is NOT a part of the human female reproductive system?
(a) Ovary
(b) Uterus
(c) Vas deferens
(d) Fallopian tube
Answer: (c) Vas deferens
48. In which of the following organisms, multiple fission is a means of asexual reproduction?
(a) Yeast
(b) Leishmania
(c) Paramoecium
(d) Plasmodium
Answer: (d) Plasmodium
49. In bifocal lenses used for the correction of presbyopia:
(a) the upper portion is of convex lens for near vision and the lower part is a concave lens for distant vision.
(b) the upper portion is of convex lens for distant vision and the lower part is a concave lens for near vision.
(c) the upper portion is a concave lens for near vision and the lower part is of convex lens for distant vision.
(d) the upper portion is of the concave lens for the distant vision and the lower part is of the convex lens for the near vision.
Answer: (d) The upper portion is a concave lens for distant vision and the lower part is a convex lens for near vision
50. Identify the food chain in which the organisms of the second trophic level are missing:
(a) Grass, goat, lion
(b) Zooplankton, Phytoplankton, small fish, large fish
(c) Tiger, grass, snake, frog
(d) Grasshopper, grass, snake, frog, eagle
(e) Tiger, grass, snake, frog
Answer: (c) Tiger, grass, snake, frog
CBSE Class 10th Science Exam Important MCQ's Answer Key FREE PDF Download |
Other Related Links
- CBSE Class 10th Science Sample Paper And Solutions By Experts
- CBSE Class 10th Science Most Repeated Questions
- CBSE Class 10th Science Important Diagrams
Comments
All Comments (1)
Join the conversation